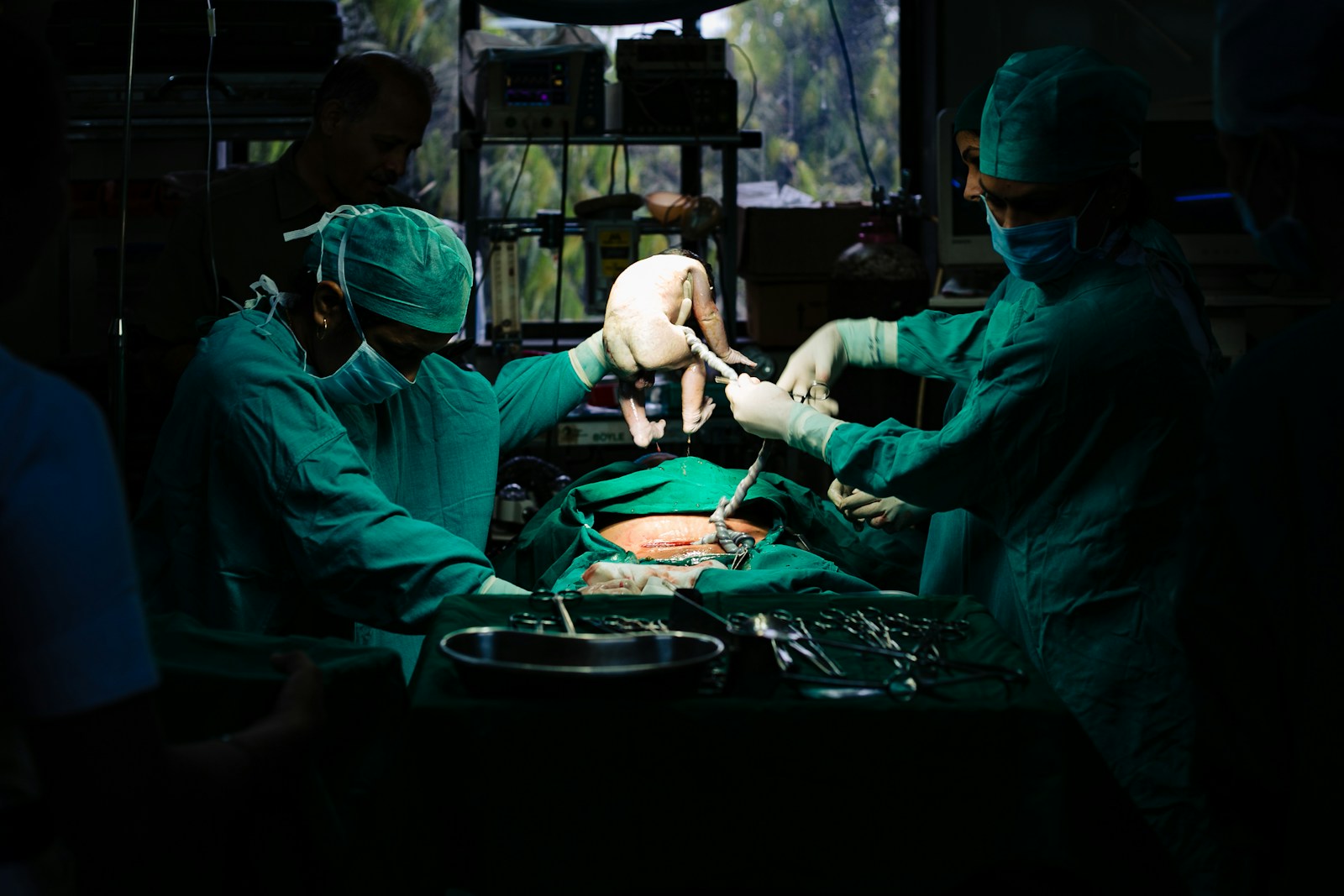
Recent advancements in neonatal care have introduced stem cell therapy as a promising treatment to protect extremely preterm infants from brain injuries. Researchers at Monash Children’s Hospital in Australia have successfully collected and reinfused umbilical cord blood cells in babies born before 28 weeks of gestation, demonstrating the feasibility and safety of this innovative approach.
Key Points at a Glance:
- Innovative Treatment: Umbilical cord blood-derived stem cells are reinfused into extremely preterm infants to potentially prevent brain injuries.
- Successful Trial: The Cord-Safe Study demonstrated that 23 extremely preterm infants received cord blood-derived stem cell infusions with no serious adverse events reported, confirming that the procedure is safe and tolerable in this vulnerable population.
- Potential Benefits: Stem cells may reduce inflammation and promote brain development, decreasing the risk of conditions like cerebral palsy.
Preterm birth, defined as delivery before 37 weeks of gestation, poses significant risks to infant health, particularly concerning brain development. Infants born extremely preterm (before 28 weeks) are especially vulnerable to brain injuries that can lead to lifelong neurological impairments, including cerebral palsy. Traditional medical interventions have had limited success in preventing such outcomes, highlighting the need for innovative therapies.
The Cord-Safe Study, conducted by a collaboration of Monash Health’s Monash Children’s Hospital, The School of Clinical Sciences at Monash University, and Hudson Institute of Medical Research, focused on infants born before 28 weeks of pregnancy. Collecting cord blood from these tiny infants, who often weigh less than 750 grams, presented significant challenges due to smaller placentas, minimal blood volumes, and complex delivery circumstances. Despite these obstacles, the team managed to successfully collect and process cord blood cells from approximately 70% of the extremely premature infants enrolled in the study.
The reinfusion of these autologous (the infant’s own) cord blood-derived stem cells occurred within 10 days of birth. Stem cells are known for their anti-inflammatory and immunomodulatory properties, which can be particularly beneficial in reducing the inflammatory reactions that harm the developing brain. Additionally, they may encourage growth and repair processes, providing a protective effect against brain injuries.
One notable case from the study involved Billie Garey, born at 24 weeks of gestation. Billie received her own stem cells following a small brain bleed and has shown improved outcomes, exhibiting only mild cerebral palsy affecting her left lower limb. Her parents attribute her remarkable progress to the stem cell treatment, noting that she is meeting developmental milestones and leading an active life.
The success of this feasibility study paves the way for larger international trials. As cell therapies are increasingly being evaluated for neuroprotection and neuroregeneration in young children, this research positions Australia at the forefront of innovative neonatal care. The project’s lead researcher, Associate Professor Atul Malhotra, stated that this breakthrough offers hope for parents and medical professionals dealing with the complex challenges of extremely premature birth, potentially changing the landscape of neonatal care for generations to come.
While the findings are promising, further research is necessary to establish the long-term efficacy and safety of stem cell therapy in preterm infants. Future studies will aim to determine optimal dosing, timing, and delivery methods to maximize therapeutic benefits. Nonetheless, this pioneering work represents a significant step toward improving outcomes for the most vulnerable infants, offering a potential treatment option where previously … .